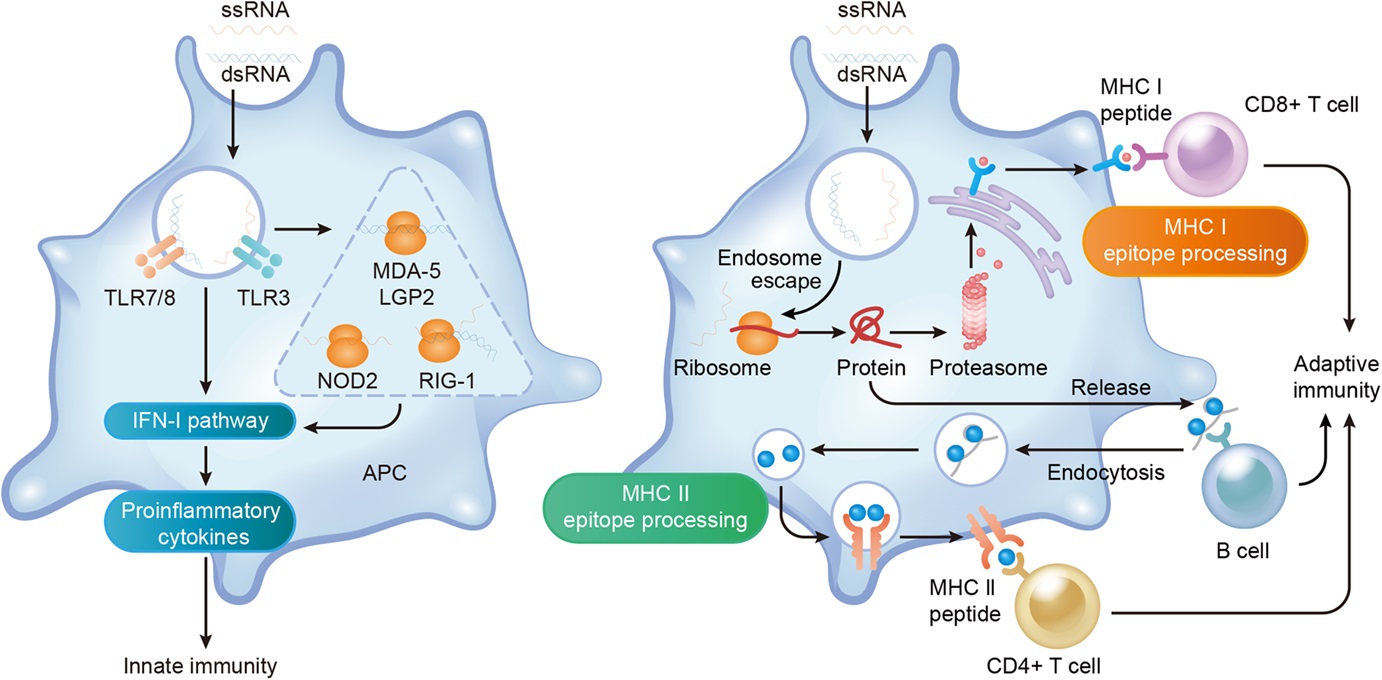
Clinical trials are studies conducted to check if a new medication, treatment, or procedure is safe for humans and how effectively it can cure their condition. Professionals conduct these studies to enhance and improve people’s lives. Clinical trials have successfully developed new treatments for fatal diseases such as cancer.
Clinical trial studies can be very challenging. Experts need to consider many aspects to make it a success. From the selection of the patient to the completion of the study, the path is not linear. Instead, it has many difficult patches that the Principal Investigator must pass. Every country has its own laws and regulations that regulate clinical trials. But the common characteristics of reputable clinical trials include respect for participants’ privacy, strong scientific evidence, independent committee oversight, compliance with relevant laws, etc. Not all clinical trials are the same, but here are common aspects that you must consider before starting a clinical trial.
Table of Contents
TogglePurpose of the clinical trial
Make sure you know the purpose of the clinical trial you are conducting. You must have a clear idea about what doctors want to learn out of it and ensure that their goals align with your procedure and expectations from the trial. Depending on the phase in which they are conducted, clinical trials aim to answer some particular questions and analyze specific criteria. Be open about the results of the clinical trials, and do not hide what you should reveal. Otherwise, it is a direct question mark on your credibility and eligibility.
Consider the ethical concerns of a trial
Clinical trials must preserve the dignity of the participants. Therefore, ethical concerns in research are an important consideration. Some of the ethical concerns in research include informed consent, the social and clinical value of the study, and the selection of subjects based on scientific goals. These trials expose the subject to risk, so they should be conducted when the risk is justified. The trial results must be shared and made available for the benefit of the medical community at large. An independent review panel should be available to evaluate the results to eliminate the chances of biases interfering with the results and efficacy of a drug or treatment. All the clinical trial participants must know the gravity of their predicament and all the risks involved. They must know the purpose, benefits, methods, and risks. Participants need to be aware of how they will benefit from the research and its relation to their situation.
Decide the country in which the trial will take place
If you are a sponsor, you must decide on the location where the study will take place. Apart from the laws governing clinical trials, other factors also influence your decision to select a country. You have to consider the patient population, the cost of the trial, the time it will take, among other things. In cases where it is mandatory to conduct a trial at a specific location, sponsors bear these factors in mind before proceeding. After selecting the country, you must choose the particular sites where you will conduct the study and see that you have all the facilities needed for successfully completing the trial. Also, ensure that the site staff is equipped with the necessary skills, knowledge, and competencies to aid clinical trials.
Develop your clinical trial team
The competencies of your clinical trial team in an in-house study can make or break your efforts. Therefore, the staff is an important consideration before starting. Looking at the end goal of your research and how it will proceed over time, you should have a staff that can aid you at all levels during your study.
Clinical trials are not for the faint of hearts or those who lack the courage to undergo the arduous and challenging process. Therefore, the team you put together should be aware of the difficulties and must ensure their availability for the long haul. Members leaving the team during the trial can be detrimental to the success and progress of the study. Not all members will have the same knowledge and understanding of the therapeutic aspect of your research. But make sure the team lead has a solid knowledge base in this regard.
Consider the budget and regulatory requirements
Clinical trials are not cheap! You must know this before you embark on this mission. They become even more expensive in totality when abandoned in the middle. The cost of the trial and the budget should be available to the sponsors ensuring they will stick to the cause till the end. It’s even better when sponsors actively participate in the whole process and give their input about how much everything will cost.
Before giving their consent, sponsors must understand the regulatory requirements ensuring that a relevant authority has approved the drug or the treatment. Their understanding of the regulatory environment surrounding the therapeutic area while having a good relationship with the agencies can give a necessary push to the study.
Risks and potential benefits
Clinical trials are risky. They are meant to test a new treatment not available on the market yet. So regardless of the scientific studies and the conclusions drawn from them, some results and effects on the body will be unknown. The researchers are also unsure if the treatment will work when tested on humans. During the early trials, the focus is not to study the effectiveness of the treatment. However, the researchers must be able to anticipate the benefits and the risks involved in the study. In a regulated trial, medical ethical committees evaluate the trial’s design to ensure that the benefits justify putting a human being in a risky situation.
No trial is without risk. Without any doubt, all medications have side effects. In the case of clinical trials, you don’t even fully know about the risks, as they are yet to unfold throughout the study. So, a realistic approach to the study is essential to eliminate any biases.
Conclusion
Clinical trials are complicated, time-consuming, and expensive. Experts need to consider these factors before starting a clinical trial study. The participants must be aware of the benefits and risks of the study. They should be able to decide without any pressure. The team must not give them any inflated hopes or dreams of a proper recovery. There is always an element of surprise because even researchers may not know how the treatment will affect the patient’s health.