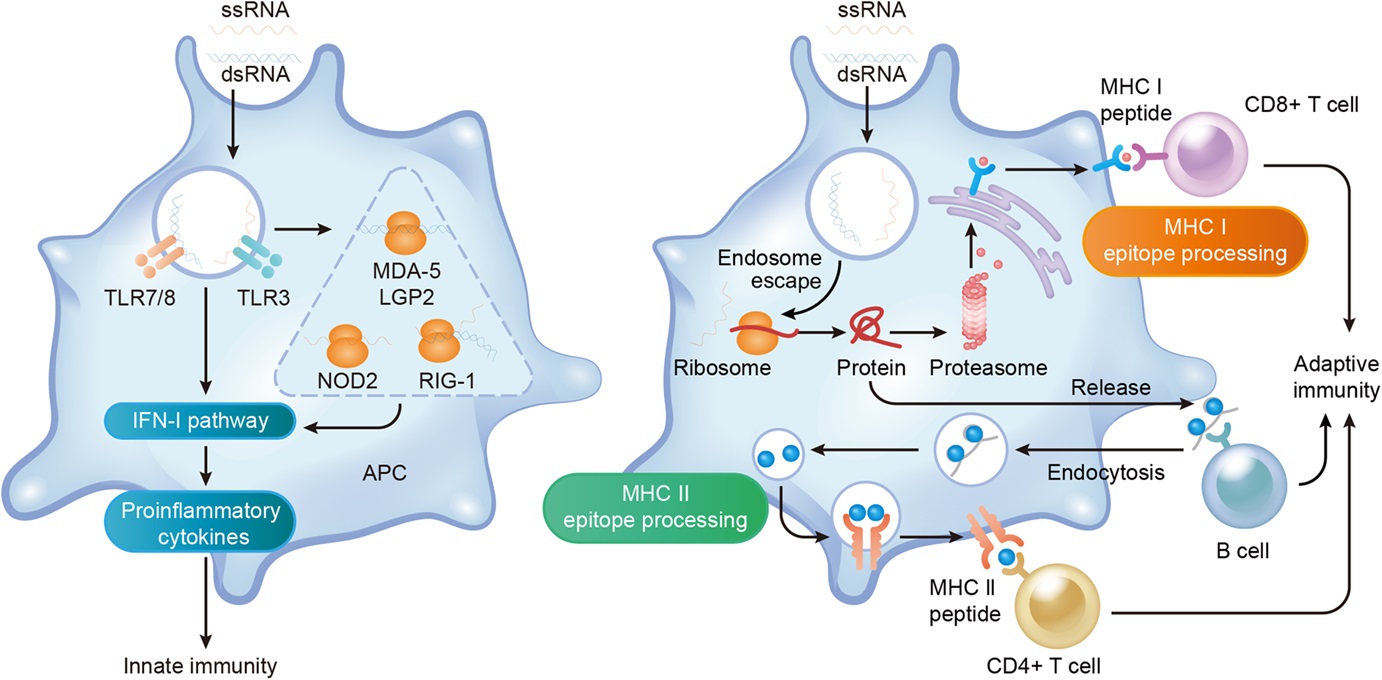
New York, May 10 (IANS) US scientists have produced a first-ever gene-edited calf that is resistant to a bovine viral diarrhoea virus (BVDV), and may help reduce reliance on antimicrobials against cattle disease.
Cattle worldwide face major health threats from a highly infectious BVDV that decades of vaccinations and other precautions have failed to contain.
If follow-up research confirms efficacy, the gene-editing approach offers long-term potential to reduce antimicrobial and antibiotic use in the cattle industry, said the researchers including from University of Nebraska-Lincoln (UNL), the US Department of Agriculture (USDA), and a Minnesota-based private company.
The BVDV devastates the bovine immune system and can cause severe respiratory and intestinal harm to infected beef and dairy cattle, said veterinary epidemiologist Brian Vander Ley, an associate professor in the UNL’s School of Veterinary Medicine and Biomedical Sciences.
Vaccines have failed against the highly mutable nature of BVDV as well as its highly virulent strains.
To control the virus, the researchers used gene editing to change the small number of amino acids that lead to BVDV vulnerability, while keeping the rest of the protein, CD46, unchanged.
“Our objective was to use gene-editing technology to slightly alter CD46 so it wouldn’t bind the virus yet would retain all its normal bovine functions,” said Aspen Workman, a scientist with the ARS US Meat Animal Research Center (USMARC) in Clay Center, Nebraska.
A gene-edited calf named Ginger was born on July 19, 2021, and was transported to UNL a week later for close monitoring. Throughout, Ginger has remained a “bright, healthy calf,” normal both physically and behaviorally, which included a week with a BVDV-infected dairy calf that was shedding the virus in great volume.
The research findings are published online in the journal PNAS Nexus.
Ginger is a Gir, a tropically adapted cattle breed used to develop Brahman cattle in North America. Follow-up research will require experimental replication in other cattle breeds. Ginger also will be monitored through pregnancy, if it occurs.
If the gene-editing approach proves viable, it could potentially reduce the cattle sector’s use of antimicrobials, Vander Ley said.
In utero calves are especially vulnerable to BVDV infection. If they survive, they can remain infected for life, repeatedly spreading the virus to other cattle.
“They show up as normal cattle, but really, they’re shedding a tremendous amount of virus. They’re the ‘Typhoid Marys’ of BVDV spread,” said Vander Ley.
“The most successful version of the future that I can see is one where we don’t have to deal with antimicrobial resistance because we just don’t use that many antimicrobials,” he said. “That’s better for everyone. That means that we have eliminated the cause of a lot of the antimicrobial use and we’ve eliminated that expense for livestock producers,” Vander Ley said.
–IANS
rvt/svn/